What’s bugging the gut in OCD?

A new review that came out yesterday in the journal Depression and Anxiety explores yet another link between a psychiatric disorders and the gut bacteria. Obsessive Compulsive Disorder (OCD) is characterized by “recurrent intrusive thoughts (obsessions) and ritualistic behaviors aimed at reducing distress (compulsions)”. Although an relatively unexplored area, the authors highlights a few interesting examples of studies that suggest a connection.
In one study from 2014, the authors used a chemical to induce a form of OCD-like behavior in mice. The behavior included moving differently that normal in an open-field, stereotypic turning, and burying marbles(!) The mice were initially divided into 3 groups: one pretreated with a probiotic, one pretreated with with Prozac, and finally one pretreated with saline (i.e. control group). Then all 3 groups were injected with the OCD-inducing chemical. After 4 weeks the OCD-like behaviors were significantly reduced in both the probiotic group and the Prozac group compared to the control group.
In another study, 25 otherwise healthy humans were divided into two groups to receive either a probiotic or a placebo for 30 days. The people were scored before and after the treatment using a number of systems examining anxiety, stress, and depression. It was shown that the people in the probiotic group improved on three sub-scores of the observed parameters, one of them being “obsessive compulsive”.
This is absolutely preliminary, but nevertheless another interesting piece in the mind-gut-bacteria-puzzle! Let’s keep digging!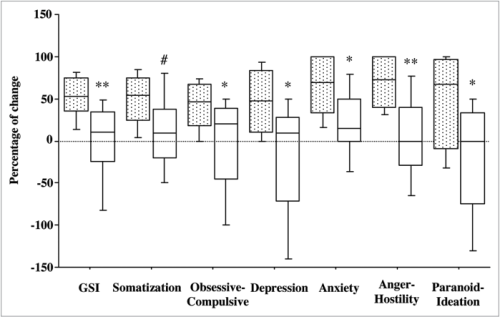
Gut bacteria affect stroke outcome
This is obvious in the gut where the immune system is constantly challenged by the billions of bacteria occupying our intenstines. Certain types of cells work to regulate the immune system and make sure it doesn’t go berserk when it’s not needed. These cells are called regulatory T cells or simply Treg.
A new elaborate study points to a connection between the state of the intestinal flora and the outcome of strokes. In mice with an altered intestinal flora, the damage following an induced ischemic brain injury was reduced significantly compared to controls. Here’s a summary of the experiments and results:
- Male mice were treated with antibiotics (AC – amoxicillin and clavulanic acid) for 2 weeks. One group of mice were housed with so-called seeder mice that carry an AC-resistant flora. Because mice are coprophagic – yuck – the bacterial gut flora is quickly transferred. After two weeks, the result was 2 groups of mice; one with an altered flora (ACSens), and one with a normal flora (ACRes) but both treated with AC. This was done to control for antibiotics side effects.
- After 2 weeks, the overall diversity of the bacterial gut flora had decreased in the ACSens mice. Proteobacteria were now dominant, and normal players like Firmicutes and Bacteroidetes were depleted.
- Both groups of mice were now subjected to a so-called middle cerebral artery occlusion (MCAO) mimicking a stroke. After 3 days, the size of the infarct (tissue damaged by stroke) was measured, and a 60% reduction was seen in the ACSens mice. The sensory and motor function of the mice was also assessed at days 3 and 7 after MCAO, and this showed a similar picture in which ACSens mice were able to perform tasks faster.
- An increase in number of Treg cells was observed in the small intestine of ACSens mice, and this was accompanied by a decrease in number of another type of T cell (γδ T cells), known to be aggrevate ischemic injury by secreting a hormone called IL-17.
- The entire setup was repeated in genetically altered mice that don’t have this type of T cells. The difference between ACSens and ACRes was abolished suggesting that the neuroprotection induced by gut dysbiosis is conveyed through these cells.
- Using another mouse model, in which cells express a fluorescent protein that can be converted from the color green to red by UV light exposure, they illuminated the small intenstine with UV light, induced a stroke, and observed how red (i.e. intestinal) T cells migrated to the meninges of the brain.
So what does all of this mean? Well, it would appear that following a stroke, immune cells from the intestines travel to the brain to clean up the mess. Depending on the state of the intestinal flora, the profile of these cells can vary. If the flora is disturbed (dysbiosis), the bacterial priming of the immune system causes the number of Treg cells to increase and number of γδ T cells to decrease. This apparently leads to less neuroinflammation and tissue damage following a stroke. This is of course peculiar as we tend to consider diversity good and dysbiosis bad. But in this case, it seems two wrongs do indeed make a right!
Big question is of course how we translate this to treatment perspectives for stroke patients. First of all, this was all carried out in mice, so we don’t know if the results translate directly to humans. But it is another, mind-bogglingly fascinating example of the gut-brain-microbiota axis that is becoming increasingly evident in research…
[NB: This post was originally published on the Tumblr used for the first phase of the exhibition project Mind the Gut. The tumblr has now been integrated into the exhibition webpage – here.]


